Introduction to Steam and Steam system.
Introduction
What is Steam?
 Steam is the evaporated gas molecules of water at a set boiling temperature and a given set pressure.
Steam is the evaporated gas molecules of water at a set boiling temperature and a given set pressure.
The most frequent method to explain this phenomenon is through the demonstration of a boiling kettle. Hence, when there is heat applied to the kettle and the temperature reached 100-degree Celsius (or 212-degree Fahrenheit) then the chains between the water H2O molecules stars to break and evaporate through the air; thus produced Steam. Similarly to the boiler’s fundamental mechanism, when the heat is applied to the contained system, steam is also produced. The pressurized steam will then travel through the pipe-lines system to where it is needed to be. Furthermore, due to its origin of water, steam is inexpensive as well as versatile to many usages within the industrial industry.
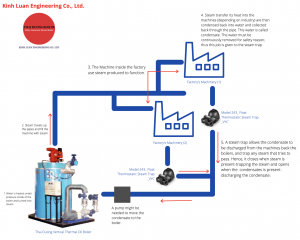
The Steam and Consedate Loop
The relationship between Steam and Pressure.
Steam is created when water is heated to a boiling point of 100 ̊C (212 ̊F); hence the heat energy is absorbed and break the bonds between the water molecules to create gas (in this case steam). However, a unique feature of steam is that, unlike water, it can reach further than 100 ̊C (212 ̊F) at atmospheric pressure or 0 PSIG. Hence, as the boiling temperature increases the steam pressure increases. Therefore, if steam is generated at a pressure higher than the atmospheric pressure then the boiling point has passed 100 ̊C (212 ̊F).
Additionally, steam does not lose its temperature when the heat has reduced. It only condensates back from a gas to a liquid form.
What is Saturated Steam?
Saturated Steam is the steam generated from the boilers while in the presence of boiling water. When additional heat is later added to the saturated steam, to increase its temperature, then the saturated steam is now turned into Superheated Steam. Superheated Steam is used in power generation and saturated steam is generally used for heating. During this heating process, the saturated steam continuously releases its energy and condensed it back into the water.
- Heating Properties: The heat (at boiling point) required to turn a liquid into a gas is known as Latent Heat. Latent heat releases energy through steam during heating. Since steam is very efficient in transferring heat to other processes. It allows the steam/water molecules to surround any surfaces it needs to transfer the heat into. As mentioned before, during the heating process, the steam will continuously release heat and condensates back into the water. Simultaneously, the water will be transferred back into the boiler and reheated to become steam (referred to the “The Steam and Consedate Loop” – ab0ve).
Where is Steam used for?
Steam can be used for a variety of processes within every industry. Such as the pharmaceutical industry, food & beverage, waste-water treatment facilities, and many more.
Example(s) of Kinh Luan Engineering’s customers:





